Our Medical Translation Methodology
Transparent processes. Subject-matter expertise. Defensible quality assurance.
Built for Precision and Regulatory Accountability
We translate life science content to your exact specifications—from brand voice and patient literacy levels to regulatory compliance. Our multi-step quality process delivers precision and consistency every time.
8-Step QA PROCESS
BEFORE TRANSLATION
Define Your Needs
We review your document type, target audience, regulatory context, and required literacy level to define project specifications before translation begins.
Expert Team Selection
We will select the translation and editing team that best suits the subject matter and expertise needed for your project.
Glossary and Style Guide
We establish or integrate client-specific glossaries and terminology controls to ensure consistency across studies, sites, and ongoing projects.
TRANSLATION & REVIEW
Translation Platform
All translations are completed within our secure, encrypted translation environment with terminology validation, version control, and integrated QA checkpoints. This ensures consistency, traceability, and controlled document handling.
Editing
A second medical linguist independently reviews the translation for clinical accuracy, terminology consistency, readability level, and adherence to project specifications.
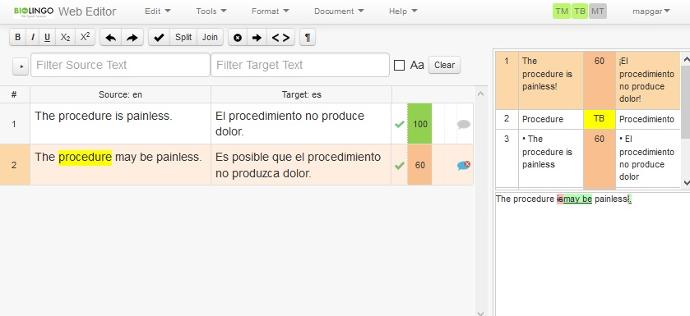
Proofreading
A final proofreader conducts a quality control review for formatting integrity, linguistic precision, and typographical accuracy prior to release.
Desktop Publishing
We ensure layout accuracy, text expansion management, and formatting consistency to preserve the integrity of patient-facing and regulatory documents.
Delivery and Follow Up
Final documents are delivered securely along with documentation required for institutional or IRB review. Ongoing terminology updates are incorporated into future projects to maintain long-term consistency.
Ready for compliant medical translation?
Get Your Free Quote
Project scoped and priced in 24 hours.
Medical Subject-Matter Qualification Standards
Our medical translation team is selected and evaluated through a structured, multi-layer qualification system designed for clinical accuracy and regulatory reliability.
Ongoing Quality Oversight
Peer-reviewed performance evaluations
AI-assisted quality assurance validation
Structured client feedback integration
Continuous terminology and compliance monitoring
Native Speakers with Cultural Expertise
Master complex medical terminology across language pairs
Convey your message with natural accuracy and nuance
Understand cultural beliefs and health practices of diverse communities
Vetting Process
Verification of academic and professional credentials
Documented medical and life science translation experience
Subject -specific written assessment prior to onboarding
Our Top Medical Translators
9.8/10 average rating across 5000+ informed consent translation projects.
Work with our top medical translators.
Start Your Project
We support over 50 clinical, technical, and CMS-integrated file formats.
Contact us for a complete list.
Microsoft Office
- DOC / DOCX
- XLS / XLSX
- PPT / PPTX
Websites
- HTML
- XHTML
- PHP
Localization
- XML
- JSON
- STRINGS
Desktop Publishing
- MIF
- IDML
Images
- JPG / JPEG
- TIF / TIFF
- BMP
- PNG
- GIF
Subtitles
- SRT
- TTML
- VTT
Technical Writing
- DITA XML
- MANUAL XML
CMS Connectors
- MAGENTO
- WORDPRESS
- GIT
- DRUPAL 8
- JOOMLA 3
Language Coverage and Locale Precision
We provide medical translation in more than 40 languages, with regional localization options (e.g., U.S. Spanish, LATAM Spanish, Spain Spanish). We guide clients in selecting the appropriate locale for their patient population and regulatory context.
Most Popular Language Combinations
American English to Neutral Spanish translation
American English to Brazilian Portuguese translation
Haitian French to American English translation
American English to Ukrainian translation
Certified Translation Accuracy, Every Time
The certificate of translation accuracy will include:
Who translated it and their qualifications
A declaration that the translation is complete and accurate
Which document was translated and the language pair
The translator's name, signature, and date
A notary seal
We can provide a notarized Certificate of Translation Accuracy for any medical translation. A separate reviewer verifies accuracy and completeness, and the certificate confirms the translation hasn’t been changed since signing. Our QA process supports IRB submissions and regulatory audits.
Life Science Translation FAQs
How does BioLingo safeguard protected health information?
BioLingo follows HIPAA web-hosing protocols on a secured IT infrastructure. Additionally, all our translators and vendors sign NDAs.
BioLingo follows HIPAA web-hosing protocols on a secured IT infrastructure. Additionally, all our translators and vendors sign NDAs.
How does BioLingo manage medical terminology?
BioLingo's translation platform integrates custom glossaries, style guides, and translation memories. Linguists can see all these linguistic assets in real time. This ensures accuracy and consistency for all documents you translate with us.
BioLingo's translation platform integrates custom glossaries, style guides, and translation memories. Linguists can see all these linguistic assets in real time. This ensures accuracy and consistency for all documents you translate with us.
Why do medical translations require specialized human translators?
Medical documents are full of vital information that must be properly understood by the reader. Errors by machine translation engines or inexperienced translators could change the meaning in harmful ways.
Medical documents are full of vital information that must be properly understood by the reader. Errors by machine translation engines or inexperienced translators could change the meaning in harmful ways.
Accurate. Compliant. IRB-Ready.
Launch your next translation project today.
Get My Quote


